OUR HIV RESEARCH
- Unique Antiviral Activity -
Currently combination antiretroviral therapy (cART) can control viremia and keep it below the lower limits of detectability in the best cases, but it is not a definitive HIV cure.
In fact, a residual undetectable low level viremia would still be present and it is one of the factors that make HIV infection incurable and should be targeted. It is dependent on at least two interconnected reservoir compartments where cART has little or no effect: one being the latently infected cells that might reactivate after stimulation (HIV reservoir) and one consisting of an exiguous number of cells that continue to produce low levels of virus. Both these compartments should be addressed simultaneously, and this is the advantage offered by the FHP DDX3 inhibitors.
The innovative technology that lies behind FHP’s DDX3 inhibitors is that they not only block viral replication (Brai et al., PNAS 2016), but are also able to induce selective cell death in HIV infected and producing cells (Rao et al., Nature Communications in 2021). The inhibition of viral replication and the induction of cell death by FHP DDX3 inhibitors occur in active cells as well as those reactivated from the latent reservoirs, as largely elucidated in many assays by our independent academic partners.
Based on the evidence of activity against various resistant strains and on the ex vivo proof of concept, it could be anticipated that the FHP compounds will contrast residual viremia and HIV reservoirs in People Living with HIV (PLWHIV).
Four main effects that make FHP DDX3 inhibitors unique:
- block of the viral replication with a new ARV mechanism for which there are no resistant strains and that will contrast also residual viremia
- selective kill of the reactivated HIV infected cells leading to reservoir reduction
- selective kill of any active HIV infected cells, meaning that they will kill both the reactivated latent cells, as well as the low level cART escaping cells
- inhibition of human host factor that makes impossible for the virus to select a mutation to develop resistance to the DDX3i.
It is the combination of these four unique qualities of our compounds that will inevitably lead to a major reduction of the residual viremia as well as of the HIV circulating and hidden latent reservoirs, with a final contrasting effect on the viral rebound after cART interruption.
TRANSLATION INHIBITORS
Translation Inhibitors offer unique possibilities uncommon to most of HIV antivirals, such as a very high genetic barrier to resistance and the possibility to reduce the dormant reservoirs, paralleled by low toxicity and favourable body distribution
FHP's Translation Inhibitors As Antivirals
Our DDX3 RNA Helicase inhibitors belong to the Translation Inhibitor class of compounds that do not target viral proteins but temporarily inhibit an enzyme of our own RNA cell biology that is of vital importance for the virus during its reproduction cycle. As such there is no or very low possibility that drug-resistant strains are selected due to the low mutational rate of human proteins and the fact that the virus should make use of a host factor other than DDX3 to evade the drug activity. Furthermore, the host cells as well as the non-infected cells are not particularly affected by the inhibition of their own enzyme as they can rely on different pathways for their functions. This is confirmed by the almost absent cytotoxic effects of our compounds both in vitro and in vivo. On this basis, a low systemic toxicity is expected even for long term treatments. In addition, our Translation Inhibitor class of DDX3 helicase inhibiting compounds are also able to promote selective cell death of infected cells contributing to virus eradication.
UNIQUE MECHANISM OF DDX3 INHIBITORS IN HIV
Unlike Protease Inhibitors, the other class of compounds that act after viral DNA integration into human genome, Translation Inhibitors completely block the production of new virions by the inhibition of the human host factor DDX3 helicase that mediates viral protein translation. This way, viral components remain trapped within the cell because the synthesis of the essential polyproteins is disrupted. As such, the unsuccessful tentative formation of new viral particles inevitably leads to the accumulation of mRNA in the nucleus and of mRNA and incomplete proteins in the cytoplasm: a part of the protein material will likely be re-metabolised, but the accumulation of cellular waste ultimately activates pro-apoptotic mechanisms. Repeated failed attempts to produce new virions would inevitably accumulate even more viral waste within the host cell and ultimately promote apoptosis. As a result, it becomes clear that DDX3 inhibitors have both antiviral and reservoir reduction effect.
RESERVOIR REDUCTION
The persistence of viral reservoirs of HIV infected cells is the main barrier to HIV cure
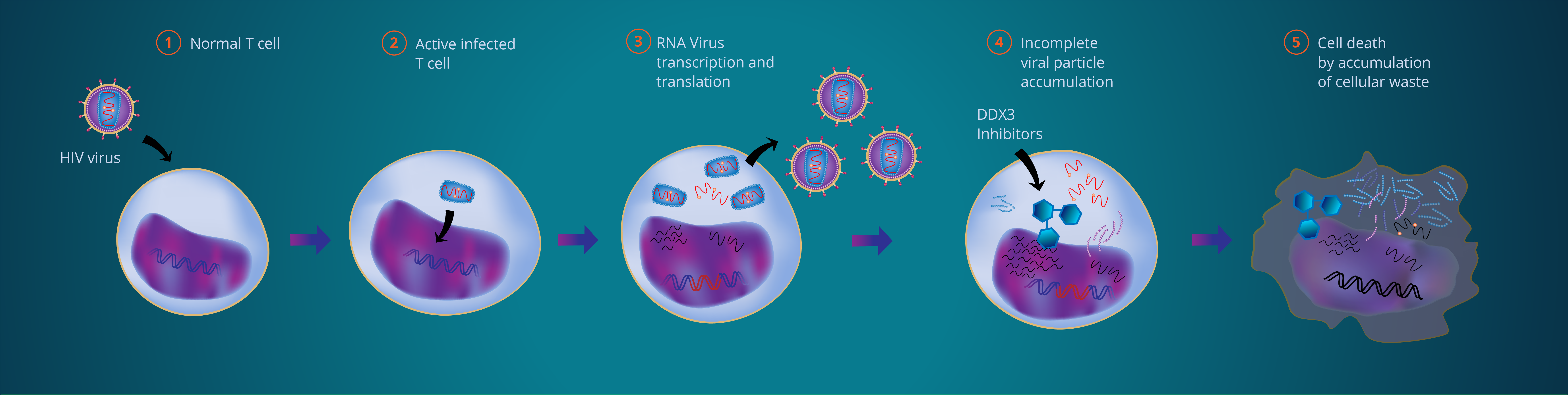
1 – An HIV virion infecting an immune cell
2 – Virus gets into the CD4+ T–immune–cell. Viral DNA is integrated in the human genome.
3- Transcription and translation of integrated viral genome take place. New virus particles are created
4 – Our small molecule compound inhibits DDX3 blocking the viral mRNA in the nucleus and its translation to proteins. Few or incomplete viral particles are assembled
5 – No new virions are released and the infected cells are eliminated by induction of cell death
HIV RESERVOIRS
Viral load in blood becomes undetectable during combination antiretroviral therapy (cART), but the virus is dormant in HIV reservoirs and can always come back. The virus can hide out in a “latent” form inside certain cells of the immune system such as CD4+ T cells, macrophages and glia, forming the so-called HIV reservoirs. Because of this latency state the HIV infected cells undergo little or no transcription, thus the immune system cannot detect and eliminate (or attack) them. Once antiretroviral therapy is stopped, these viral-reservoir cells can rapidly fuel HIV rebound. Main anatomical reservoirs are intestine, lymph nodes and the blood CD4+ T memory cells, as well as organs protected by barriers impermeable to cART, such as brain and testis.
THE ROLE OF HIV RESERVOIRS
Cells carrying latent but replication competent proviral genomes represent the true big hurdle in the treatment of HIV and in the development of curative therapies. Latently infected cells can survive for years with a very slow natural decay. As a result, forcing their elimination toward eradication of the virus is currently considered a promising solution to the problem. An efficacious approach must distinguish between healthy uninfected and infected cells and selectively kill the latest, preferably without release of new virions. The presence of HIV reservoir not only represents an odd against cART interruption, but also maintains a pernicious pro-inflammatory state in the patients that is not contrasted by cART.
RESERVOIR REDUCTION BY DDX3 INHIBITORS
Our DDX3 translation inhibiting compounds effectively block the production of new virions by the inhibition of the DDX3 helicase enzyme, a non-essential co-factor of the host cell’s protein apparatus, hijacked by the virus, during its translation from proviral integrated DNA (scheme image – step 3). This way, viral components remain trapped within the cell because the export of viral unspliced mRNAs and the synthesis of the essential polyproteins is strongly inhibited. As such, the tentative formation of new viral particles from proviral DNA inevitably leads to the accumulation of unspliced mRNA in the nucleus and spliced mRNA and polyprotein material in the cellular cytoplasm (scheme image – step 4). Only a part of the protein material will likely be re-metabolised, while the rest will accumulate as intracellular waste, known to be a pro-apoptotic mechanism. Repeated failed attempts to produce new virions will inevitably accumulate ever more viral waste within the host cell and ultimately promote apoptosis (scheme image – step 5).
SHOCK AND KILL
DDX3 inhibitors can be part of a Shock & Kill approach - currently the most promising therapeutic strategy for HIV cure
SHOCK AND KILL STRATEGY
Even though combination Antiretroviral Therapy (cART) has been the most revolutionary HIV therapeutic approach so far, that alone is not enough to completely eradicate HIV. The Shock and Kill approach is a two-step model which aims at reactivating (Shock) the dormant HIV infected cells – the HIV reservoir – through latency reversal agents (LRAs) and subsequently eradicating (Kill) these cells by means of a combination of antiretroviral drugs, clearance by host immune system and inducer of selective cell death. . First Health Pharmaceuticals is currently participating in three multi center public-private collaboration projects on HIV cure strategies mainly focusing on Shock and Kill approach and Reservoirs Reduction. These projects, funded by Health Holland and AIDSfonds, include several high profile research groups from, Amsterdam University Medical Center (AUMC), Erasmus Medical Centre (EMC), Utrecht Medical Centre (UMC), Viiv Healthcare and other industrial partners. The projects have confirmed the potential of our Translation Inhibitor to induce selective cell death of HIV infected reactivated cells, alone or in combination with Latency Reversal Agents (LRA) in different models, including ex vivo PBMC from people living with HIV under cART and measured with gold standard assays (e.g. qVOA).
LATENCY REVERSAL
The “Shock” step of the Shock and Kill strategy consists in the reactivation of the provirus hiding in the latent HIV infected cells by means of Latency Reversal Agents (LRAs).
The target patients are the people living with HIV (PLWH) whose viral load has been suppressed below the level of treatment by effective highly active cART.
Several in vivo and in vitro studies on LRAs have investigated their ability to reactivate and reduce latent reservoirs, alone or in combination with other compounds: so far no LRA has been found to induce reservoir reduction on top of viral reactivation when administered alone to human and neither there are successful clinical studies for Shock & Kill.
Among the most advanced LRA, toll-like receptor (TLR) activators have been shown to reactivate latent HIV infected cells. The development of TLR7 agonists is part of First Health Pharmaceuticals pipeline.
TOLL-LIKE RECEPTORS AGONISTS
First Health Pharmaceuticals has been investigating potential Shock strategies to be applied in a HIV Shock & Kill approach. Since early 2016, we started studying the potential use of Interleukins and Toll-like Receptor (TLR) agonists, which is a class of compounds that we were already intimately familiar with through our cancer research into TLR7/8.
Toll-like receptors (TLR) class of protein belongs to the family of pattern-recognition receptors (PRR). They are transmembrane receptors line able to recognize conserved molecular structures called pathogen-associated molecular patterns (PAMPs) and promote viral reactivation in CD4+T cells by serving as signaling receptors in the innate immune system. Besides their ability to reactivate latent HIV, TLR agonists also increase immune activation and promote an antiviral response. These combined properties make TLR agonists unique among the different LRAs characterized to date. First Health Pharmaceuticals’ HIV pipeline includes both DDX3 RNA Helicase inhibitors and TLR7 agonists that potentially could contrast HIV reservoir formation and maintenance, and combined can contribute to viral eradication.
Selective Cell Death Conception And Validation
The Conception of Selective Cell Death Strategy
On the 23rd and 24th of February 2016, Jan Willem Bakker and Alessia Tarditi of First Health Pharmaceuticals (FHP), had a series of email exchanges containing ideas that were key to the first conception of selective cell death of HIV infected cells treated with DDX3 inhibitors.
Scroll this page or jump in the timeline to:
Timeline of research, starting from 2015
2015
DDX3 Helicase Inhibitors
When FHP was founded, back in 2015, our goal was clear: ending HIV as we know it. This meant putting major effort on HIV cure, thus in discovering and investigating new generations of antivirals.
From the beginning, FHP core research strategy focused on Translation Inhibition, which consists in blocking the production of new virions by inhibiting the ATP-dependent RNA helicase DDX3 enzyme bypassing the resistance issue associated to targeting viral enzymes.
The FHP DDX3 inhibitors have higher specificity for RNA viruses and target the RNA binding site rather than the ATP binding one, with significant reduction of toxicities and undesired off targets mediated effects.
2016
First publication on PNAS
This investigational approach led to the discovery of the first generation of DDX3 helicase inhibitors (DDX3i) in 2015 and their antiviral activity has been disclosed via a publication on the PNAS journal in 2016. In the same year the first antiviral patent has been approved and made public.
Conception of Selective cell death
In February 2016, First Health Pharmaceuticals‘ Jan Willem Bakker and Alessia Tarditi conceived the idea that DDX3 inhibition could lead to accumulation of untranslated viral RNAs as wells as aborted proteins and other mechanisms that would have led to the selective death of infected cells (selective apoptosis). Furthermore, it was also discussed the idea that DDX3 inhibition might have induced reactivation of dormant virus and cells, due to its participation in specific intracellular pathways (undisclosed). Read more about the story of FHP’s Selective cell death strategy for HIV Cure.
2017
A new series of DDX3 Helicase inhibitors
FHP started investigating a new series of DDX3 helicase inhibitors with improved in vitro and in vivo properties which were presented less than one year later in Amsterdam at the AIDS 2018 international conference.
2018
AIDS2018
The peculiarity of the new series of DDX3 inhibitor compounds presented at AIDS2018 was the fact that they showed some selective pro-apoptosis activity towards HIV infected cells. The hypothesis emerged in 2016 was close to be confirmed and was shared and extensively discussed with KOLs and experts in the field during and after the conference. The possibility to induce selective cell death with the DDX3 inhibitors caught the eye of the HIV scientific community and led to the participation of FHP in three major multicentre projects focused on HIV cure in 2019.
2019
Selective pro-apoptotic activity confirmed
The confirmation of induction of selective apoptosis of DDX3 inhibitors in translationally relevant models, including ex vivo samples, arrived after the participation in three major multicentre projects for HIV cure:
1 | TARGET2CURE with AUMC and UMC and Health Holland partnership
2 | Genezingvooriedereenanders (ICK4HIV) with EMC, UMC, ViiV
3 | HIV Cure with AUMC and Health Holland partnership
In these projects, the induction of selective cell death and the effect on latent cells exerted by the FHP DDX3 inhibitors were tested in samples from people living with HIV representing the closest model to an in vivo study available for HIV.
Accordingly, FHP made progress in the development of a new series of DDX3 inhibiting compounds.
2020
Latency reversal activity confirmed
Not only were the selective apoptosis activity of DDX3 inhibitors confirmed, but they were also showing activity of latency reversal. The first results came out from a research study conducted by EMC in the Netherlands, and in August 2020, data were made available to the community via a preprint in BioRxiv, in line with the goal of all the participants to contribute to the knowledge around HIV cure and the progression toward the identification of a cure .
Noteworthy, the FHP DDX3i Helicase inhibitors showed the capability to induce latency reversal and selective cell death, and were also able to contrast viral replication and did not show any in vitro or in vivo toxicities.
2021
FHP´s breakthrough idea and compounds published in Nature Communications
As a result of both Latency Reversal and selective cell death induction shown by the FHP DDX3 Helicase inhibiting compound FH1321 in the EMC study, a 50% HIV reservoir reduction in PLWHIV was confirmed and, in April 2021, the paper was published in Nature Communications.
At the same year, FHP was involved in another project where new results have shown that a potential combination of DDX3 inhibitors with other compounds can maximise their effect (undisclosed).
2022
FHP´s compounds can induce cell death in actively HIV infected cells
Another effect of the compounds that contributes to HIV cure is that the induction of cell death also occurs in actively HIV infected cells, not only as part of a Shock & Kill approach as shown before. This effect would contribute to control the residual viremia in people living with HIV (PLWHIV) under cART and contrast the formation of reservoirs if the DDX3i are given soon after the infection.






